News
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a MemberSelected News

IRAM Brings Together Global Partners to Celebrate 90 Years of Standards Work
The Argentine Institute of Standardization and Certification (IRAM) marked its 90-year milestone by gathering more than 200 representatives from
ENAC Passes OECD Good Laboratory Practice Assessment With No Deviations
The National Accreditation Body of Spain (ENAC) has passed the Organisation for Economic Co-operation and Development (OECD) assessment of its Good Laboratory Practice (GLP) oversight programs with no deviations.
Standards Watch Issue 23 Highlights Safer Sustainable Plastic Packaging
The 23rd edition of the Standards Watch video series explains how plastic packaging can be made safer and more sustainable.
Germany Releases First Safety Standard for Plug-In Solar Devices
The German Commission for Electrical, Electronic & Information Technologies (DKE) will publish the world’s first product standard for plug-in solar devices in December 2025, giving
Mexico Recognizes MDSAP as Equivalent to National Medical Device GMP
Mexico has issued a new interpretation rule for the NOM-241-SSA1-2025 standard on good manufacturing practices (GMP) for medical devices, confirming that
Korea Forms National Council To Drive Automotive SDV Standards
Korea has formed a national council to advance software-defined vehicle (SDV) standards, a step meant to strengthen the country’s future position in the automotive sector.
You Can’t Replace Czech Quality: November Episode Highlights Trust in Quality Brands
The November episode of You Can’t Replace Czech Quality series explains why Czech quality remains a trusted sign of reliable products and services.
INEN and UASB Invite Public to Join International Quality Congresses
The Ecuadorian Standardization Service (INEN) and the Andean University Simón Bolívar (UASB) are inviting participants to the XVI International Quality Congress and the VII Integrated Quality Congress,
Inacal Accredits 39 Public Conformity Assessment Bodies to Improve State Service Quality
Peru’s National Quality Institute (Inacal) has accredited 39 public-sector conformity-assessment bodies to strengthen the quality, safety and reliability of state services nationwide.
Danish Version of European Standard EN 45560 Brings Circularity Into Product Design
Denmark has published DS/EN 45560, its national version of the European circular product design standard, giving companies a practical way to integrate circularity into product development from the start.
Quality Week 2025 Shows How UKAS Strengthens Its Culture of Quality
The United Kingdom Accreditation Service (UKAS) marked World Quality Week 2025 by reflecting on what quality means across the organisation and how the theme Quality:Think Differently links to its everyday work.
Dutch In Vitro Standards Committee Presents Broad Agenda for New Laboratory Standards
The Dutch In Vitro Diagnostics Standards Committee is driving a wide range of international work on new laboratory and diagnostic standards, reflecting the rapid rise of digital tools, artificial intelligence, and smarter testing systems.Global News
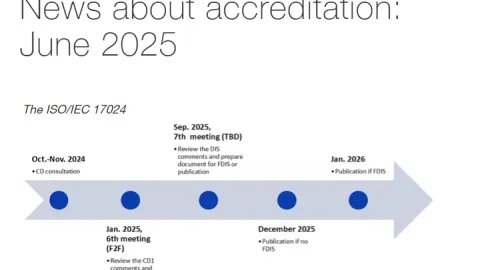
June 2025 Accreditation Roundup: ILAC-IAF Merger Advances, New IAF Documents and More
International accreditation organizations ILAC (International Laboratory Accreditation Cooperation) and IAF (International Accreditation Forum) have accelerated merger plans into a common global organization
Global Experts Gather in Australia to Advance Revision of ISO 20387 Biobanking Standard
ISO/TC 276 Biotechnology technical committee held its annual plenary in Cairns, Australia, bringing together experts from 41 countries to focus on revising ISO 20387, the international standard for biobanking.
IAF PL 3 Issue 5 Refines Process for Adding New Areas to Global Accreditation System
The International Accreditation Forum (IAF) has published Issue 5 of IAF PL 3, its policy on the structure of the Multilateral Recognition Arrangement (MLA) and how new areas can be added.
How IEEE’s Cybersecurity Certification Supports Connected Medical Devices
The IEEE Standards Association is advancing efforts to safeguard connected medical devices through its IEEE Medical Device Cybersecurity Certification Program.
ISO 42005 Sets Global Framework for AI System Impact Assessment
ISO and IEC have released ISO/IEC 42005:2025, a new international standard that helps organizations assess how AI systems affect individuals and society.
New Decision Tree Explains Food Safety Risk Levels in FSSC Version 2.0
Foundation FSSC has published a new decision tree to help food businesses determine whether a control measure should be classified as a Critical Control Point (CCP) or an Operational Prerequisite Program (oPRP).
IAFP Honors Sobeys With 2025 Black Pearl Award for Food Safety Excellence
Sobeys Inc. has been named the 2025 recipient of the Black Pearl Award by the International Association for Food Protection (IAFP),
IAF Outlook May 2025 Spotlights UNIDO Partnership and Regional Developments in Africa
The International Accreditation Forum (IAF) has published the May 2025 edition of its newsletter IAF Outlook, spotlighting major partnerships, technological advances, and accreditation efforts across regions.
Tech Giants Back New Standard to Clarify End-of-Life Security Timelines
A group of leading tech firms has proposed a new standard, called OpenEoX, to streamline how companies disclose product end-of-life and end-of-support dates.
ISO Launches Pilot Licensing Model With New Duties for Certification Bodies
The International Organization for Standardization (ISO) has launched a pilot of its Differentiated Licensing Model (DML), introducing major changes to how standards are accessed, priced, and controlled.
Sixteenth Meeting of WG 29 Advances ISO 9001 Revision
ISO/TC 176/SC 2/WG 29, the working group responsible for revising ISO 9001, met online for its sixteenth session on May 14, 2025.

