News United States of America
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a MemberSelected News

ANSI Invites Experts To Lead U.S. TAG For Automation Standards
The American National Standards Institute (ANSI) is inviting experienced automation professionals to consider leadership roles in the U.S. Technical Advisory Group (TAG) to ISO/TC 184/SC 4, which contributes to international standards for automation systems and industrial data.
NIST Launches AI Agent Standards Initiative To Address Security And Interoperability
The National Institute of Standards and Technology (NIST) has launched an AI Agent Standards Initiative to address security, identity, and interoperability challenges as AI agents become more widely used.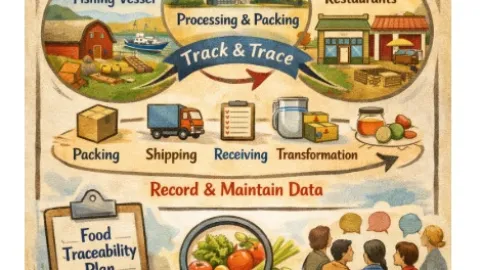
FDA Issues New Guidance On Food Traceability Rule
The U.S. Food and Drug Administration (FDA) has released new guidance to help companies comply with its Food Traceability Rule, clarifying how the requirements apply across the food supply chain.
NIST Announces 2025 Baldrige National Quality Award Recipients
Two U.S. health care organizations have been selected to receive the 2025 Malcolm Baldrige National Quality Award, the nation’s only presidential honor for performance excellence.
SQF Institute Names Finalists for 2026 Excellence Awards
The SQF Institute has announced six finalists for its 2026 SQF Excellence Awards, recognizing professionals who advance food safety through auditing and site leadership under the Safe Quality Food program.
ANSI And BSB Introduce ANSI Bharat Compliance Platform For Indian Market
The American National Standards Institute (ANSI) and BSB Edge, a compliance technology company serving regulated industries in India,
Zaire Garcia Wins Terry Burgess Assessor of the Year Award
Zaire Garcia has won the Ninth Annual Terry Burgess Assessor of the Year Award from the ANSI National Accreditation Board (ANAB) for his leadership in assessments and strong commitment to mentoring others.
ANAB Seeks National Experts For Personnel Credentialing Committees
The ANSI National Accreditation Board (ANAB) is inviting nominations for two national committees that oversee personnel certification and certificate accreditation.
How AFDO Review Service Speeds Up Retail HACCP Plan Approvals
The Association of Food and Drug Officials (AFDO), a nonprofit association focused on food safety and regulatory collaboration, offers a national Retail HACCP Plan Review Service to help streamline approvals of specialized food processes.
FDA Aligns U.S. Device Rules With ISO 13485, Replaces QSR With QMSR
The U.S. Food and Drug Administration (FDA) has replaced its long-standing Quality System Regulation (QSR) with the new Quality Management System Regulation (QMSR), changing the legal framework for quality management in the U.S. medical device sector.
Winter Issue of ANSI in China Newsletter Highlights 2025 Developments
The American National Standards Institute (ANSI) has released the Winter 2025 edition of the ANSI in China Newsletter, outlining recent standards, policy, and regulatory developments linked to U.S. and China engagement.
Baldrige Foundation Announces 2026 Leadership Award Recipients
The Foundation for the Malcolm Baldrige National Quality Award has announced the 2026 recipients of its Leadership Awards,Global News

ISO 14001 Final Draft Approved For Publication
The Final Draft International Standard (FDIS) of ISO 14001 has been approved, paving the way for publication in mid April.
IATF Establishes Legal Entity, Ending Ad Hoc Structure
The International Automotive Task Force (IATF) has established a new legal entity, IATF AISBL, marking a shift from its previous structure as an ad hoc group of automotive manufacturers and their national industry associations.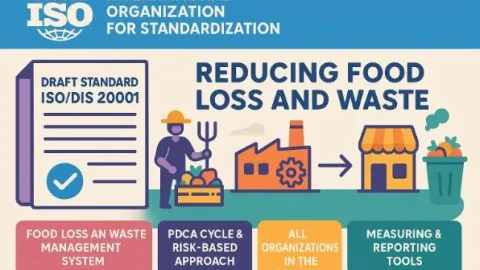
Have Your Say On Food Waste Management: ISO 20001 Survey
Harper Adams University is inviting professionals across the food chain to take part in a survey on ISO 20001, a proposed international management system standard designed to help organisations systematically reduce food loss and waste.
GOTS Version 8.0 Released With Expanded Due Diligence And Environmental Controls
Global Standard has released Version 8.0 of the Global Organic Textile Standard (GOTS),
Global Quality Infrastructure Index 2025 Report Ranks Germany First as Certification and Accreditation Expand
The 2025 edition of the Global Quality Infrastructure Index (GQII) has been released, presenting updated rankings and new data on the development of national quality infrastructure systems.
BRCGS Announces 2026 EMEA Award Winners At Connect Europe
BRCGS has announced its 2026 award winners during BRCGS Connect Europe in London, recognizing individuals and organizations serving the Europe, Middle East and Africa (EMEA) region and adjacent markets.
Food Safety Culture Becomes a Formal Certification Requirement Under SQF Edition 10
When SQF Edition 10 launches in March, food safety culture will be required and auditable as part of certification.
Revised ISO 10012 Reframes Metrology As A Management System
The revised ISO 10012 standard has been published, positioning metrology as an integral part of quality management systems rather than a standalone technical activity.
ISO 14091 Under Review To Ensure It Meets Climate Adaptation Needs
The International Organization for Standardization (ISO) is conducting a systematic review of ISO 14091 to determine whether it still meets the needs of organizations addressing climate change risks.
Six Changes Reshape ISO 17020 Requirements For Inspection Bodies
The 2026 revision of ISO/IEC 17020 introduces structural and conceptual updates that will affect how inspection bodies operate, particularly in areas such as independence, impartiality, complaints handling, and management systems.
IFS Strengthens Audit Consistency With Good Audit Practices Guideline Version 2
IFS has released Good Audit Practices Guideline Version 2 to support auditors in delivering more consistent, high quality and effective audits across all IFS Standards.

