Blogs
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a Member
ISO 13485 Alone Does Not Make You IVDR Ready
A valid ISO 13485 certificate alone does not mean an in vitro diagnostic medical device manufacturer is ready for the In Vitro Diagnostic Regulation (IVDR).
90 Day ISO Certification Debate Hinges on Readiness Not Speed
Can a company achieve ISO certification in 90 days?
Seven Decisions That Shape Your Future In Quality
The quality leader you become in five years is shaped by the decisions you make now.
In Aerospace, Severe Consequences Leave No Room For Speed Over Precision
Quality failures in aerospace carry disproportionate consequences, even when they are rare.
Clause 6 On Planning Separates Strategy From Paperwork
Clause 6 on planning in ISO 9001 and IATF 16949 reveals whether a company treats risk as a strategic tool or as a paperwork exercise.
MSA vs ISO GUM: Why One Cannot Replace the Other
MSA (Measurement System Analysis) and the ISO GUM (Guide to the Expression of Uncertainty in Measurement) both deal with measurement variation, but they serve different roles in quality and laboratory decisions.
8 Questions to Ask Before You Adopt an AI Quality Management Tool
Adopting AI in quality management requires a structured, risk-based approach, especially in regulated environments.
Leadership Must Give Quality a Voice
Quality assurance must be empowered to speak up and act when risks are identified, even if that means stopping production.
Quality Leaders Must Decide Which Issues Deserve A CAPA
If quality leaders had unlimited time and money, they could investigate every issue with a full CAPA (corrective and preventive action).
AFNOR Study Pinpoints Weaknesses in ISO 9001 and ISO 14001 Certified Organizations
A new AFNOR Certification study, drawing on nearly 20,000 audit reports, shows that organizations certified to ISO 9001 and ISO 14001 perform strongly in operational planning.
Lack Of Hands On Experience And Structural Concerns In ISO Standard Development
A recent reflection by healthcare quality expert Dr. Sambhu Chakraborty questions whether there is sufficient real world implementation experience among the people who write ISO standards.
Most Wasteful Standard Revision In History? ISO 9001 Update Under Fire
The ongoing revision of ISO 9001 could become the most wasteful standard revision in history, according to Mark Kaganov.
Constructive Or Inspection-Obsessed Auditors, Which One Builds Real Value?
ISO audits are shaped less by qualifications and more by the auditor’s mindset.
How To Get Regulatory Standards For Free
When a regulation requires compliance with a “voluntary” standard, companies may be able to access that standard for free through a special online platform, the ANSI IBR Portal managed by the American National Standards Institute (ANSI).
Lean Principles Show Why AI Still Needs People
Lean principles help organizations get more value from AI by strengthening continuous improvement and supporting human judgment rather than replacing it.
Five Major Auditor Challenges Accredited Certification Bodies Face
Accredited certification bodies are facing five recurring challenges that are increasing pressure on audit delivery as demand for accredited audits continues to grow.
Hype Vs Reality In The Requirement Changes Of The Revised ISO 9001
Claims that the revised ISO 9001 introduces major new requirements are overstated,
Zero Audit Findings May Seem Impressive but Say Little About Real Performance
Zero audit findings may seem like proof of strong control, but two aviation quality professionals argue that such results often lack substance and can hide real risks.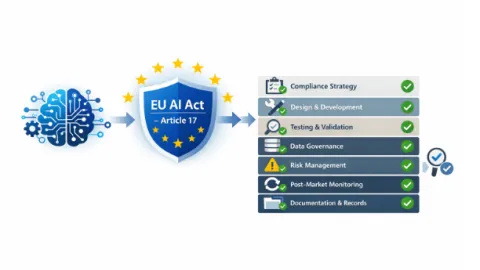
How EN 18286 Guides Quality Management Compliance Under EU AI Act
As the European Union’s Artificial Intelligence Act (EU AI Act) comes into effect this summer, providers of high-risk AI systems will be required to operate a documented quality management system (QMS) covering the full lifecycle of their systems.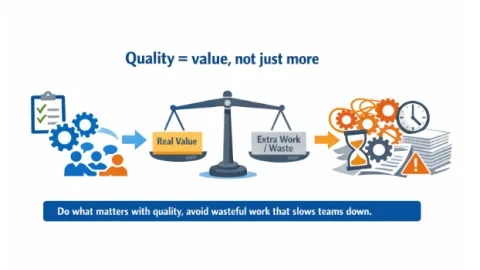
When Balance Is Lost, Quality Becomes Inefficient
Quality efforts can become inefficient when they are over-applied or poorly managed, creating waste, delays, and friction instead of value.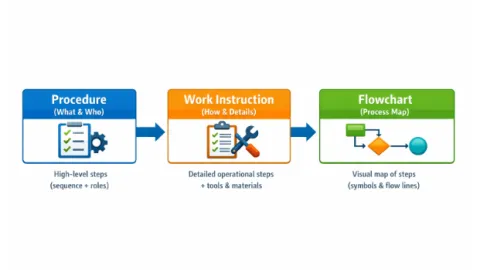
Choosing Between Procedure, Work Instruction, Or Flowchart In Your QMS
Choosing between a procedure, a work instruction, or a flowchart is a practical decision that directly affects how clearly a quality management system (QMS) works in everyday use.
How the IATF FAQ Update Changes Certification Scope for Aftermarket Parts
Following the IATF updates published in November 2025, certification bodies have begun clarifying how those requirements will be applied in practice.
Behind Every ISO Standard Stand Dedicated Experts Who Rarely Get Credit
ISO standards rely on the work of dedicated experts whose contributions often go unnoticed, despite their global impact on trust, safety, and quality.

