News Medicine / Pharmaceutical
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a MemberSelected News

Hoan My International Hospital Laboratory Earns ISO 15189 Accreditation
The medical laboratory of Hoan My International Hospital has received ISO 15189:2022 accreditation from the National Accreditation Bureau (BoA),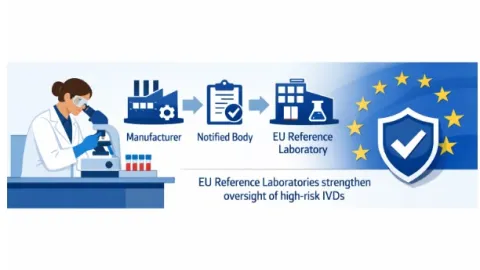
EU Expands Laboratory Oversight for Highest Risk In Vitro Diagnostic Devices
The European Union (EU) has expanded oversight of Class D in vitro diagnostic medical devices under Regulation (EU) 2017/746, known as the In Vitro Diagnostic Regulation (IVDR).
BIS Seeks Public Feedback on Draft Standards for Medical Devices and Healthcare
The Bureau of Indian Standards (BIS), through its Medical Equipment and Hospital Planning Department,
NATA Seeks Experts in Software as Medical Devices and AI for Human Pathology Advisory Committee
The National Association of Testing Authorities, Australia (NATA) is inviting expressions of interest to join its Human Pathology Accreditation Advisory Committee,
Denmark Aligns Accreditation Rules for Referral Laboratories and Specialists With ISO 15189
Denmark has revised its national accreditation rules for the use of referral laboratories and external specialists to align with the Danish version of ISO 15189:2022 for medical laboratories.
FDA Aligns U.S. Device Rules With ISO 13485, Replaces QSR With QMSR
The U.S. Food and Drug Administration (FDA) has replaced its long-standing Quality System Regulation (QSR) with the new Quality Management System Regulation (QMSR), changing the legal framework for quality management in the U.S. medical device sector.
NEN Seeks Experts for Clinical Research Standards Committee
The Netherlands Standardization Institute (NEN) is inviting experts to join its Clinical Research standards committee to help shape international standards for clinical investigations of medical devices.
Clearlab Earns MDSAP, ISO 13485 and EU MDR Certifications for Contact Lenses
Clearlab, a manufacturer of vision correction products and lens care solutions, has obtained combined certification under the Medical Device Single Audit Program (MDSAP), ISO 13485, and the European Union Medical Device Regulation (EU MDR).
EU Medical Device Database EUDAMED Opens First Mandatory Modules
The European Commission has published the first four functional modules of EUDAMED, the EU database for medical devices and in vitro diagnostic devices.
Italy Launches Accreditation for ISO 7101 Healthcare Quality Systems
Italy has opened accreditation for certification bodies that audit healthcare organizations against UNI ISO 7101:2024, the standard for quality management in health services.
IEI Secures TFDA QMS Approval and ISO 13485 Certification
IEI Integration Corp, a Taiwan-based provider of medical and industrial computing systems,

