Blogs
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a Member
Standardization 2.0: How Integrated Leadership Turns Standards Into Practice
Many companies still view standards and certifications as proof of success, but such an approach is hollow without active leadership.
8 Reasons Why HSEQ Management Systems Meet Their Demise
Diana Parisi, Director and Principal Consultant at JUMP Consulting, shares a sharp and creative reflection on why many HSEQ (Health, Safety, Environment, and Quality) management systems fail.
Debunking the Top Five Myths About Accreditation
Accreditation is often seen as rigid, bureaucratic, or focused on perfection, but in reality, it plays a vital role in ensuring trust, quality, and accountability across industries.
Real-Time Oversight Makes Store Brands Leaders in Food Safety
Store brands are now setting the benchmark in food safety and transparency thanks to real-time data sharing and built-in safety design,
Why Carbon Pricing Needs Cooperation, Not Competition on Data Standards
Robert Eccles, Chair of KKR’s Sustainability Expert Advisory Council and former Harvard professor,
Ghost Processes: Turning Hidden Risks Into Opportunities for Improvement
Many organizations run on two management systems: the official one in the manual and the real one people use every day.
Don’t Panic! Four Ways to Ace an Audit Without Preparation
An unplanned audit can feel like a nightmare, but Gillet Friedrich, Managing Director and consultant at OPTIQUM, reminds readers that it’s no reason to panic.
In Audits Like in Courtrooms, Evidence Makes or Breaks the Case
A blog post by Perfect ConneXions, a business consulting and services company based in Chesterfield, England, draws a striking parallel between ISO audits and courtroom trials.
Small Companies, Big Lessons for Food Safety Professionals
Food safety and quality expert Jarmain Mkandla, Director of Quality at Safenel Group, shares a personal perspective urging professionals not to focus solely on big-name employers.
A Future Without Food Audits: Certification Set for Major Shift
Food safety and quality expert Ir. Cornelis van Elst, owner of QAssurance, predicts that traditional food certification models will soon be replaced by continuous, data-driven assurance systems.
Watch How Auditors Assess Multisite Quality Systems under IATF 16949
The International Automotive Oversight Bureau (IAOB) and the Society of Motor Manufacturers and Traders (SMMT) released a new video explaining how multisite audits are performed under IATF 16949,
Europe’s Precautionary vs American Risk-Based Food Safety Approach
Distinct approaches to food safety have led to clear differences between European and American food standards,
Quality and Security Must Converge in ICT: Lessons from the CrowdStrike Failure
A massive software outage in July 2024 exposed how fragile the global information and communications technology (ICT) ecosystem can be when quality and security are managed separately.
Ten Blunders That Can Ruin an ISO Audit
Diana Parisi, Director and Principal HSEQ Consultant at JUMP Consulting, offers practical advice on what not to do during an ISO audit.
Quality Mindset: The Path to Turning Everyday Work Into Leadership
Quality is not about strict rules or certifications but about the way you are wired to think and act,
AI Set to Transform Asset Management But Human Oversight Remains Key
The idea of a quality system that can detect and respond to equipment issues before they affect product quality is
Reasons Why AI Alone Cannot Replace Human Role in Management Systems
Artificial intelligence can support the creation of management systems,
Stop Pointing Fingers at Quality, Each Department Must Do Its Part
Quality is often blamed when problems occur in a company, becoming the main focus of criticism whenever issues arise.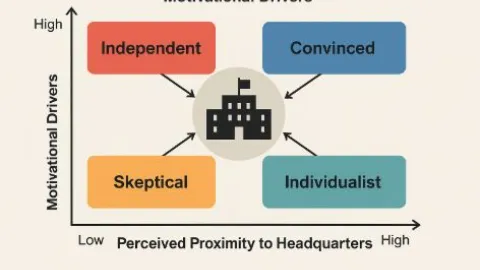
The Ways Motivation and Proximity Shape Views on Multi-Site QMS
A new study by Swedish researchers Marcus Hedberg, Ida Gremyr, and Jan Lenning from Chalmers University of Technology,
Why Recalibration Can Make Measurements Worse, Not Better
Many organizations believe frequent recalibration guarantees better measurements,
From Stress to Success: The Right Preparation for FDA Audits
An FDA audit doesn’t have to be a stressful event if companies are prepared with strong quality systems, complete documentation, and a proactive compliance culture.
Psychological Safety Is Key to Honest and Effective Audits
Auditors often face pressure to soften their findings or avoid raising uncomfortable issues,
How Companies Benefit from Participation in Standardization Committees
Active participation in standardization committees can help companies shape industry developments,

