News United States of America
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a MemberSelected News

GLOBALG.A.P. Compliance Guide Published for U.S. Fruit and Vegetable Farms
A national interpretation guideline has been published to help United States producers meet the requirements of the Integrated Farm Assurance (IFA) v6 GFS standard for fruit and vegetables,
NIST Standard Enables Accurate Dosage of New Anticancer Drugs
The National Institute of Standards and Technology (NIST) has developed the first U.S. standard for measuring the radioactivity of actinium-225, a key ingredient in a new class of anticancer drugs.
Digital Transformation and Smart Standards Take Center Stage in USNC Current Spring 2025 Edition
The Spring 2025 edition of the USNC Current, published by the American National Standards Institute (ANSI) and the U.S. National Committee (USNC) of the International Electrotechnical Commission (IEC),
USA Replaces AI Safety Institute With New CAISI To Promote Innovation
The Trump administration is replacing the U.S. AI Safety Institute (AISI) with the newly formed Center for AI Standards and Innovation (CAISI).
ANSI May 2025 Report Maps Progress and Gaps in Drone Safety Standards
The American National Standards Institute (ANSI) has released the May 2025 Gaps Progress Report to track the standardization status for unmanned aircraft systems (UAS).
ANAB Gains Global Recognition for Aerospace Quality Accreditation
The ANSI National Accreditation Board (ANAB) has expanded its international recognition under the International Accreditation Forum (IAF) multilateral recognition agreement (MLA) to include the aerospace industry’s IAQG ICOP sub-scope, as of April 23, 2025.
Cleaner Homes, Stronger Batteries: New ASTM Standard for battery-powered cleaning devices
ASTM International is developing a new standard to improve how battery-powered cleaning devices are tested in real-world conditions.
Canada–US Standard Sets Clear Method to Measure Hydrogen's Environmental Impact
A new binational standard sets a shared method to report how hydrogen is made and how much carbon is released in the process.
FDA Extends Deadline for Front-of-Package Nutrition Label Proposal
The U.S. Food and Drug Administration (FDA) has extended the comment period for its proposed front-of-package (FOP) nutrition labeling rule until July 15.
WELL for Residential Certification Brings Holistic Health to Housing
WELL for Residential, developed by the International WELL Building Institute (IWBI), is the first holistic, third-party verified certification program designed specifically to address health and well-being in all types of housing.
ANSI Seeks U.S. Experts to Help Shape Future Automation Standards
The American National Standards Institute (ANSI) is calling for experts to join the U.S. Technical Advisory Groups (TAGs) to ISO Technical Committee 184 and its subcommittees SC 4 and SC 5.
CSQ Opens Public Consultation on Revised Cannabis Safety and Quality Certification Program
Cannabis Safety and Quality (CSQ) has launched a public comment period for its newly revised certification program, aiming to simplify the process for cannabis operators across the supply chain.Global News

Shift to Green Coding Responds to Rising Energy and Environmental Pressure
Green coding, the practice of designing and writing software that uses less energy, is becoming an important part of modern software development.
IATF Releases New Updates to Rules 6th Edition and IATF 16949
The International Automotive Task Force (IATF) has approved Stakeholder Communiqué SC-2025-003, announcing new Sanctioned Interpretations and Frequently Asked Questions for both the Rules 6th Edition and the automotive quality standard IATF 16949.
Codex Commission’s 48th Session Confirms Updates Across Core Food Safety Standards
The Codex Alimentarius Commission adopted major changes to international food-safety rules during its 48th session in Rome from November 10 to 14.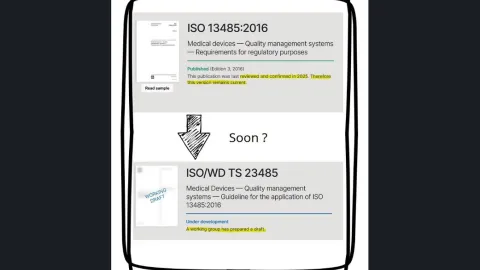
ISO 13485 Application Guide, TS 23485, Under Way After Standard Confirmed as Current
A new guide on how to apply ISO 13485 is moving through development shortly after the standard was reconfirmed in its 2016 version (no revision will be done), despite remaining unaligned with the Annex SL structure.
A Different Approach: China Strengthens Oversight of Certification Bodies from 2026
China has issued new regulations that directly target accredited certification bodies that ignore international requirements, and the new rules will take effect on January 1, 2026.
ISO and IEC Develop Global Standard to Embed Ecodesign in Medical Devices
The International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) are developing a new global standard to formally integrate ecodesign into the development of medical devices.
IFS Releases Updated Guideline for Effective Foreign Body Management
International Featured Standards (IFS) has issued an updated Guideline for an Effective Foreign Body Management,
Germany Heads Most ISO Secretariats But China Rapidly Gains Ground
Germany remains the world’s top country in international standardization, holding 17.1% of all ISO secretariats, according to the 2025 International Standardization Barometer published by the German Institute for Standardization (DIN).
Europe Now Conditionally Accepts FDA Inspections Conducted Abroad
The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have entered a new stage of cooperation under their Mutual Recognition Agreement (MRA) for Good Manufacturing Practice (GMP) inspections.
New International Standard in Development for Microbial Cultures and Probiotics
Work has begun within ISO to create a global standard that defines how microorganisms used in food and feed, such as bacteria, yeasts, and fungi, should be characterized and quality assured.
BS 30480 Launched as the World’s First Standard on Suicide Awareness in the Workplace
The world’s first standard dedicated to suicide awareness and prevention in the workplace, BS 30480, has been released by the British Standards Institution (BSI).

