News Swaziland
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a MemberSelected News

Quality Austria and ÖVQ Announce Finalists for DACH Quality Awards 2026
Quality Austria and the Austrian Association for Quality Assurance (ÖVQ) have announced the finalists for the 2026 Quality Awards, recognizing leading professionals, young talents, and teams across the DACH region (Germany, Austria, and Switzerland).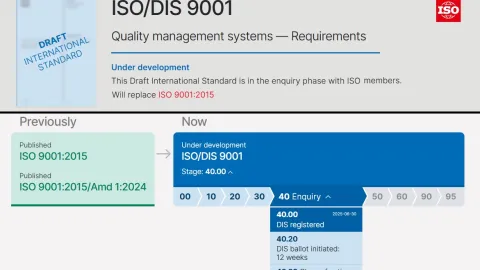
Switzerland Invites Public Comments on ISO 9001 Draft Revision
Switzerland is calling on experts and stakeholders to review and comment on ISO/DIS 9001:2025,
Switzerland Opens Comment Period on First European Standards for Digital Product Passports
The first six draft standards for the European Digital Product Passports (DPPs) are now open for public comment in Switzerland until September 5, 2025.Global News

ISO Launches ISO 14092 Climate Adaptation Planning Standard For Local Governments And Communities
The International Organization for Standardization (ISO) has published ISO 14092:2026, a new International Standard that sets clear requirements and guidance for climate change adaptation planning by local governments and communities.
ISO 9002 Revision: Second Working Draft Completed
The International Organization for Standardization (ISO) has completed the second Working Draft of its ISO 9002 revision, marking further progress in updating the guidance to reflect changes in ISO 9001.
FSSC 22000 Version 7 Nears Release with Major Food Safety Updates
FSSC 22000, a global food safety certification scheme developed by the Foundation FSSC, is moving toward Version 7, a major update that will reshape how food businesses manage safety, align with new global requirements, and prepare for future industry demands.
ISO 45001 Second Committee Draft Version Highlights Safety Culture and Workplace Wellbeing
The revision of ISO 45001 has advanced with the publication of the second version of the first Committee Draft, maintaining the standard at the Committee Draft stage and reflecting feedback from the first international enquiry.
ISO 23662 on Vegetarian and Vegan Foods Enters Periodic Review
ISO 23662:2021, the international standard that defines criteria for vegetarian and vegan foods, has entered its periodic review phase, giving stakeholders the opportunity to decide whether it should remain unchanged or be revised.
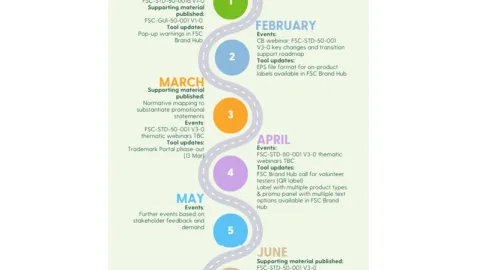
PEFC Circular Materials Task Force Established, Experts and Stakeholders Invited to Apply
The Programme for the Endorsement of Forest Certification (PEFC) has established its PEFC Circular Materials Task Force and is inviting nominations for membership by March 18, 2026.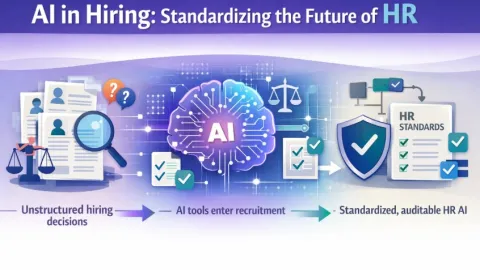
ASTM Develops WK91420 Guide for Artificial Intelligence in HR
ASTM International is developing a new guide, WK91420, to set clearer standards for artificial intelligence (AI) in recruitment and hiring, aimed at reducing bias and improving fairness.
ISO 9001 Revision Advances After Review of 1,700 DIS Comments
The revision of ISO 9001 has moved into a decisive stage, with international experts completing the review of comments on the Draft International Standard (DIS) and confirming that the new edition is on track for publication in September 2026.
ISO Launches ISO 14019 Series With New Standards Supporting Validation and Verification of Sustainability Information
The International Organization for Standardization (ISO) has published the first three standards launching the ISO 14019 series,
ISO 18060 Sets Global Rules to Measure Tourism Environmental and Social Impact
ISO 18060, a newly published international standard from January 2026, sets out a global framework to help tourism organizations measure their environmental, social, human, and economic impacts in a consistent and comparable way.
IFS Introduces Local Check to Support Small-Scale Food Producers
International Featured Standards (IFS) has introduced IFS Local, a new annual standalone food safety check designed to help small-scale and artisanal food producers demonstrate