News United States of America
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a MemberSelected News

ANSI Launches Resource on How Standards-Driven Public-Private Partnerships Shape Standards
The American National Standards Institute (ANSI) has introduced a new webpage detailing five models of Standards-Driven Public-Private Partnerships (SD-PPPs) and 19 related use cases.
Medical Device Industry at Midpoint in Transition to FDA’s ISO 13485-Based QMSR
A year ago, the FDA adopted ISO 13485 into its Quality System Regulation, rebranding it as the Quality Management System Regulation (QMSR).
"Beyond Celiac" Unveils New Trademark for Gluten-Free Products
Beyond Celiac, a leading gluten-free association in the United States, has introduced a new trademark following the release of Issue 4 of the BRCGS Standard.
ANAB Seeks Experts for Personnel Certification and Certificate Accreditation Committees
The ANSI National Accreditation Board (ANAB) is inviting nominations for its Personnel Certification Accreditation Committee (PCAC) and Certificate Accreditation Program Accreditation Committee (CAPAC).
NIST Boosts Textile Recycling Efforts with Comprehensive NIR-SORT Database
The National Institute of Standards and Technology (NIST) has developed the Near-Infrared Spectra of Origin-defined and Real-world Textiles (NIR-SORT), a free and downloadable database designed to improve textile sorting efficiency at recycling centers.
ANAB Announces Inaugural Conformity Assessment Symposium for 2026
The ANSI National Accreditation Board (ANAB) will host its first Conformity Assessment Symposium (ACAS) on January 19-20, 2026, at the Gaylord Palms Resort & Convention Center in Orlando, Florida.
Eurofins Introduces GMP Certification Program for Dietary Supplements
Eurofins Healthcare Assurance has launched a new Good Manufacturing Practice (GMP) certification program to help dietary and food supplement companies meet U.S. regulatory standards.
SCC and A2LA Launch First Dual Accreditation Program for North America
The Standards Council of Canada (SCC) and the American Association for Laboratory Accreditation (A2LA) have introduced a dual accreditation program to help testing and calibration laboratories achieve seamless cross-border compliance.
Private Brands Increase Market Share, Approach Quarter of Retail Sales
Private brands now account for nearly a quarter- 24% - of retail sales in ten main product sectors, according to recent research by Numerator.
FDA Overhauls ‘Healthy’ Food Labeling with Stricter Criteria
The U.S. Food and Drug Administration (FDA) has issued a final rule updating the criteria for the “healthy” nutrient content claim on food packaging.
ANSI Invited Experts to Shape the Future of Sustainability Standards
The American National Standards Institute (ANSI) called for experts on January 3, 2025, to join a Virtual Technical Advisory Group (VTAG) and contribute to the revision of ISO Guide 82:2019,
Applications Open for 2025 Baldrige Award, the Highest U.S. Honor in Organizational Excellence
The National Institute of Standards and Technology (NIST) has announced the 2025 Baldrige Award Criteria, signaling the start of the application process for the nation’s highest recognition for organizational performance.Global News

EASI Model Becomes First Sustainability Governance Scheme Accredited by Accredia
The EASI Model has been introduced as the first governance scheme accredited by Accredia and open to independent certification.
Core Medical Device Safety Standard IEC 60601-1 Overhaul Puts Software, Connectivity and AI in Focus
The International Electrotechnical Commission (IEC) has launched a major revision of its foundational medical device safety standard, IEC 60601-1.
Geneva Meeting on Global Plastic Pollution Treaty Ends Without Agreement
The Intergovernmental Negotiating Committee (INC-5.2) met in Geneva from August 5 to 15 to finalize a global, legally binding treaty on plastic pollution.
IAF Issues Framework for Tackling Risks of Counterfeit Certificates
The International Accreditation Forum (IAF) has released IAF ID 17:2025, a new informative document that provides a framework for managing risks related to counterfeit certificates.
SGS Launches Guidances to Support Transition to New Versions of ISO 9001 and ISO 14001
SGS has released two new guidance resources to help organizations prepare for the upcoming revisions of ISO 9001 and ISO 14001, both scheduled for release in 2026.
Revised ISO 30500 Standard Boosts Sustainable Sanitation in Non-Sewered Areas
The International Organization for Standardization (ISO) has released a revised version of ISO 30500, the international standard for non-sewered sanitation systems.
ISO 22002 PRP Standards on Food Safety Overhauled With New Umbrella and Sectoral Updates
The ISO 22002-100:2025 standard on prerequisite programmes (PRPs) for food safety introduces a common framework for hygiene and safety practices across the food, feed, and packaging supply chain, forming the new foundation of the ISO 22002 series.
DAkkS Submits Formal Legal Fixes to GLOBAC Code of Conduct and Governance Documents
The German Accreditation Body (DAkkS) has formally submitted its proposed amendments to the GLOBAC Code of Conduct, Constitution, and General Rules, aiming to eliminate legal barriers preventing its accession.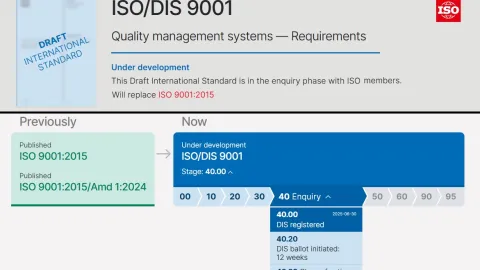
ISO 9001 Revision Shall Reach Ballot Stage
The revision of ISO 9001 has reached a new milestone, as the Draft International Standard (DIS) is set to enter a 12-week ballot period.
IFS Releases Updated Food 8 and PACsecure 3 Doctrines with New Audit Clarifications
IFS has released two updated normative documents: version 4 of the IFS Food 8 Doctrine and version 2 of the IFS PACsecure 3 Doctrine.
Draft of ISO 11135 for Medical Device Sterilization Under Review
A revision of ISO 11135, the global standard for ethylene oxide sterilization of medical devices, is now in the Draft International Standard (DIS) stage and under review by ISO member bodies.

