Blogs
Please note that you have to be a registered member with paid membership in order to see full articles.
Become a Member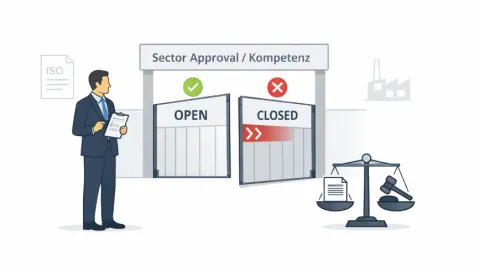
Is DAkkS Applying Auditor Competence Rules Too Strictly?
Certification expert Jörg Roggensack has criticized how the German accreditation body DAkkS interprets competence requirements for management system auditors, warning that the approach could lead some auditors to lose their ability to work in certain sectors.
ISO 9001 Can’t Save The Planet: Climate Change Expansion Weakens Quality Principles
If the revision of ISO 9001 is expected to address climate change, why should it not also solve poverty, hunger, disease, or guarantee free education and decent wages?
New Normal In Quality Shifts Focus From Order To Organizational Resilience
Quality is entering a “new normal” where traditional approaches no longer match the complexity of modern organizations.
ISO 9001 Certification in 10 Days? Possible, but Only under Strict Conditions
An ISO 9001 certification can be achieved in just 10 days, but only in rare cases.
Could Today’s Quality Professionals Pass This 1987 QA Exam?
A decades-old professional exam for quality managers has drawn attention after resurfacing online, raising the question of whether today’s professionals could still answer its questions.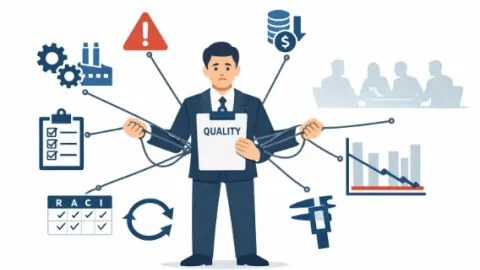
QA Managers Often Leave Their Roles Early But Companies Can Change That
Quality managers are often unhappy in their roles, writes Bruce Hutain, a quality assurance professional with over two decades of experience in such roles.
Rethinking The Future Of Audits: Remote Experts, AI Support And Augmented Reality
As auditors face growing pressure from high workloads and an aging workforce, Jörg Westphal of Hellmund Personalberater explores new ways of combining experience and technology to support the future of auditing.
ISO 13485 Alone Does Not Make You IVDR Ready
A valid ISO 13485 certificate alone does not mean an in vitro diagnostic medical device manufacturer is ready for the In Vitro Diagnostic Regulation (IVDR).
90 Day ISO Certification Debate Hinges on Readiness Not Speed
Can a company achieve ISO certification in 90 days?
Seven Decisions That Shape Your Future In Quality
The quality leader you become in five years is shaped by the decisions you make now.
In Aerospace, Severe Consequences Leave No Room For Speed Over Precision
Quality failures in aerospace carry disproportionate consequences, even when they are rare.
Clause 6 On Planning Separates Strategy From Paperwork
Clause 6 on planning in ISO 9001 and IATF 16949 reveals whether a company treats risk as a strategic tool or as a paperwork exercise.
MSA vs ISO GUM: Why One Cannot Replace the Other
MSA (Measurement System Analysis) and the ISO GUM (Guide to the Expression of Uncertainty in Measurement) both deal with measurement variation, but they serve different roles in quality and laboratory decisions.
8 Questions to Ask Before You Adopt an AI Quality Management Tool
Adopting AI in quality management requires a structured, risk-based approach, especially in regulated environments.
Leadership Must Give Quality a Voice
Quality assurance must be empowered to speak up and act when risks are identified, even if that means stopping production.
Quality Leaders Must Decide Which Issues Deserve A CAPA
If quality leaders had unlimited time and money, they could investigate every issue with a full CAPA (corrective and preventive action).
AFNOR Study Pinpoints Weaknesses in ISO 9001 and ISO 14001 Certified Organizations
A new AFNOR Certification study, drawing on nearly 20,000 audit reports, shows that organizations certified to ISO 9001 and ISO 14001 perform strongly in operational planning.
Lack Of Hands On Experience And Structural Concerns In ISO Standard Development
A recent reflection by healthcare quality expert Dr. Sambhu Chakraborty questions whether there is sufficient real world implementation experience among the people who write ISO standards.
Most Wasteful Standard Revision In History? ISO 9001 Update Under Fire
The ongoing revision of ISO 9001 could become the most wasteful standard revision in history, according to Mark Kaganov.
Constructive Or Inspection-Obsessed Auditors, Which One Builds Real Value?
ISO audits are shaped less by qualifications and more by the auditor’s mindset.
How To Get Regulatory Standards For Free
When a regulation requires compliance with a “voluntary” standard, companies may be able to access that standard for free through a special online platform, the ANSI IBR Portal managed by the American National Standards Institute (ANSI).
Lean Principles Show Why AI Still Needs People
Lean principles help organizations get more value from AI by strengthening continuous improvement and supporting human judgment rather than replacing it.
Five Major Auditor Challenges Accredited Certification Bodies Face
Accredited certification bodies are facing five recurring challenges that are increasing pressure on audit delivery as demand for accredited audits continues to grow.

